BRAIN RESEARCH
In autistic mice, a region of the brain is out of balance
It used to be difficult to take findings from research on mice and apply them to humans – especially in the case of neurological disorders like autism that can have a variety of causes. A research team at EPFL is now breaking new ground.
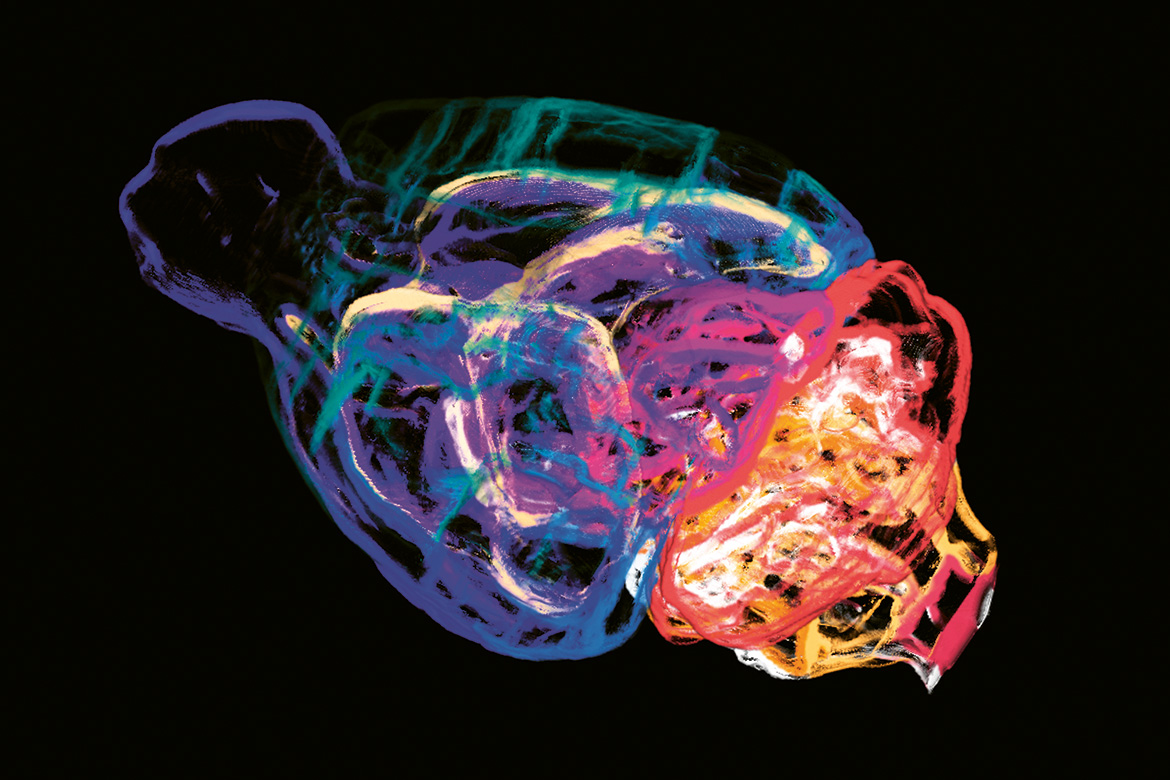
In the brains of ‘autistic’ mice, their natural default mode network is out of balance. | Image: zVg
They look just as cute as their genetically unaltered conspecifics – and yet you can notice the differences between them. Some of these mice show little interest in their own kind, for example. They don’t approach other animals or sniff at them. “Others engage in repetitive actions, such as cleaning themselves far more often than is usual”, says Valerio Zerbi, a neuroscientist at EPFL. He is describing the behaviour of mice that have been genetically modified towards autism.
16 mouse models, 16 different causes
That said: autism does not actually exist in mice. Research into humans has shown that autism spectrum disorders (ASD) have genetic causes. Today, we know of some 100 genes whose variations are linked to ASD, and research teams have been able to ‘reproduce’ some of these variations in mice. “Using the mice allows us to study the impact of these individual genetic changes”, says Zerbi. He himself is investigating their influence on certain networks in the brain: regions that act in a coordinated way in order to fulfil a specific function. He makes them visible using functional magnetic resonance imaging (fMRI). This has recently helped him to improve our understanding of autism in humans, too.
Together with an international research team, he has been investigating the so-called default mode network (DMN). In humans, it is active during certain forms of rest, e.g., when we daydream, and when we let our thoughts wander aimlessly. It becomes active in mice, for example, when they are anaesthetised for fMRI measurements. Zerbi has examined 16 mouse models – i.e., 16 different causes of autism – and compared these results with those from unmodified mice.
Zerbi discovered that the DMN functions differently in all models of autism in mice, compared with unmodified mice. The regions of this network are synchronised differently with each other, and some connections have been cut. “It’s as if this network has lost its natural balance”, he says. This happens in different ways, depending on the cause of it. None of these changes appears more than once among the 16 mouse models. But Zerbi has been able to categorise the animals into four groups in which the changes each time proceeded in the same direction. “This is the first-ever time in autism research that we have been able to find any relationship between cause and effect in the brain”.
The causes can be very different
Zerbi’s team was only able to make these findings because they investigated several mouse models – hence several causes of autism – separately. “This is exactly what autism research in humans ought to do more often”, he says. Up to now, researchers have generally done the opposite. They have tended to look for a single difference between neurotypical people and those affected by autism, without considering the possibility of multiple causes for the disorder. “This is the reason why many previous autism studies came to nothing”, he says.
No happy hormones from personal contact
This is confirmed by Camilla Bellone, a neuroscientist and professor at the University of Geneva. She is also researching into ASD with the help of mouse models, and she is currently focusing on dopamine neurons in the brain that are part of its reward and motivation system. Bellone suspects that certain causes of ASD mean that those affected are not rewarded with the happiness hormone dopamine when they engage in social interactions. “We can investigate such effects in mice and test how they might be reversed”. Bellone is confident that findings of this kind could be transferred to humans, though only if human ASD sufferers were also treated in a cause-specific manner.
As a negative example, she recalls an active agent that raised great hopes a few years ago, but then proved to be a flop. This substance was a specific brain receptor antagonist that had been developed and tested in preclinical research by using a single autism model in mice. In the clinical trials on people, it was expected to work equally well for everyone affected by ASD. “In retrospect, it is now clear that it couldn’t work”, says Bellone. This is why she would like preclinical and clinical researchers to communicate better with each other. In future, this could mean that findings from research into mice might actually be of benefit to humans suffering from ASD.