HOW IT WORKS
Arming the immune system against endometriosis
An antibody can help heal inflamed mucosal tissue outside the uterus. We look at how the Zurich start-up Fimmcyte deals with the injuries and scarring caused by endometriosis.
An antibody can help heal inflamed mucosal tissue outside the uterus. We look at how the Zurich start-up Fimmcyte deals with the injuries and scarring caused by endometriosis.
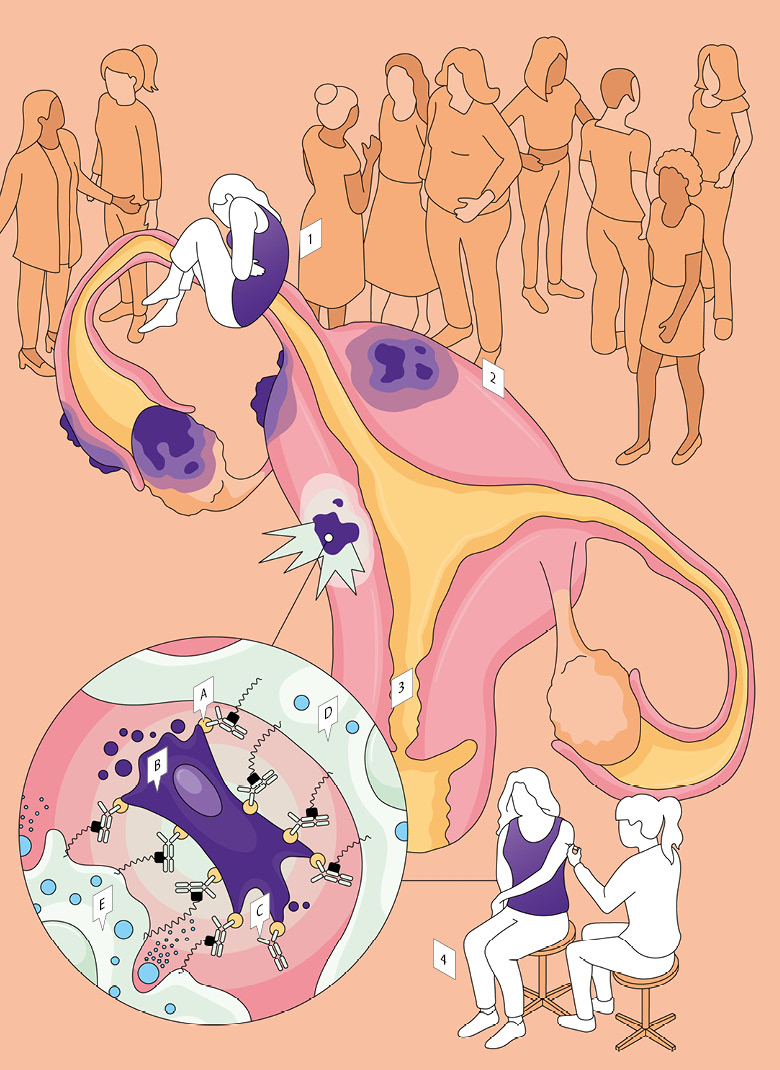
Illustration: ikonaut
E — Killer cell
D — Scavenger cell
C — Antibody
B — Myofibroblast
A — Target protein
4 — Multiple treatments necessary
The spin-off’s antibody is injected under the skin of the upper arm. The treatment has to be carried out every week for six to eight weeks. Over the course of about a year, the patient will become symptom-free. But the treatment will probably have to be repeated at a later date.
3 — Killing the sick cells
Fimmcyte is a spin-off from Zurich University and Zurich University Hospital. It’s now developing a treatment for endometriosis using immunotherapy. It has identified a target protein (A) that only occurs in specific cells in the injured tissue – the so-called myofibroblasts (B). They are produced when a wound heals. The antibody (C) matching the target protein is injected. On its way through the bloodstream, it picks up immune cells: scavenger cells (D) and killer cells (E), of which there are insufficient for the scarring in the abdominal cavity. The antibody recognises the target proteins there, binds to them and also docks onto the receptors of the two immune cell types. This triggers signals for cell death and helps to minimise and even eliminate injuries and scarring.
2 — Injuries throughout the abdominal area
The cells that leave the endometrium continue to grow and cause inflammation. This can lead to persistent injury and scarring of tissue throughout the whole pelvic and abdominal cavity. Sometimes, this even extends to the lungs.
1 — Renewed attention for an old disease
Endometriosis occurs when cells from the mucous lining of the uterus (the endometrium) spread outside it. Six to ten percent of all women suffer from it. First identified in around 1900, it’s only in recent years that it has really entered public consciousness. Those affected often experience severe menstrual pain, and it can lead to infertility. Treatments previously prescribed, e.g., aspirin, hormones or surgery (even removing the uterus itself) are not permanent and can be highly invasive.
Um Horizonte besser Ihren Bedürfnissen anpassen zu können, benutzen wir folgende Services, die Cookies auf Ihrem Endgerät speichern:
- Google Analytics
