Cellulose in a winter wonderland
Cellulose nanocrystals create colours without pigments. A microscopic image of this renewable resource shows just how talented an artist is Mother Nature.
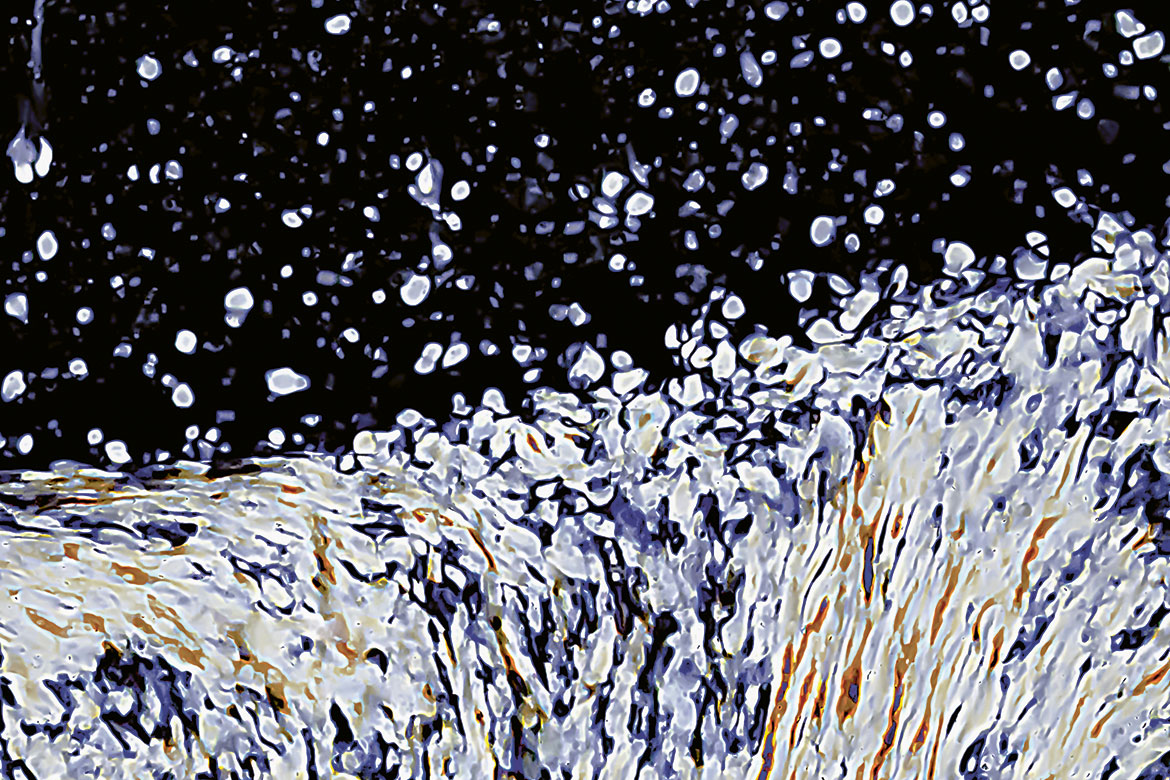
Cellulose nano-crystals under polarising light microscopy | Picture: Gwendoline Delepierre
“A colleague of mine didn’t believe that it’s a photo. He was convinced it was a painting”. But Gwendoline Delepierre – a chemist and materials scientist at the University of Fribourg – isn’t studying art. She is investigating the behaviour of cellulose nanocrystals. When she made this microscopic image, she decided to discuss with her colleagues which painter might have been responsible for it. And so they soon found a name for it: it’s now the ‘Van Gogh cellulose nanocrystal’.
“Cellulose is the biopolymer that occurs most commonly on Earth. That’s why it’s a wonderful, renewable resource”, says Delepierre. “The nanocrystals organise themselves. They are in the form of little sticks that form structures without needing any added energy”. Such as the nanocrystal shown in the image here. “I am investigating how, when and why the crystals build these structures, because they create colours without any pigments”. Incidentally, if you add salt to the sample in the photo, it turns a brilliant blue. Delepierre loves her research, because her findings might enable us to create renewable materials with intense colours.
This photo was made with a polarisation microscope. Delepierre says this object of her research “looks as if it were snowing – as if the crystals were trickling down and creating their structures. Or as if the cellulose were a wave, spraying foam upwards”. This photo awakens all kinds of associations. And it does exactly what good art is supposed to do: it makes us pause and ponder, allowing space for us to reflect on our own interpretations. And yet this seemingly expressionistic work was created using very precise techniques. “That’s what made the structures and their beauty visible in the first place”, says Delepierre. It’s a nanotechnological aesthetic – but it’s like a painting.




